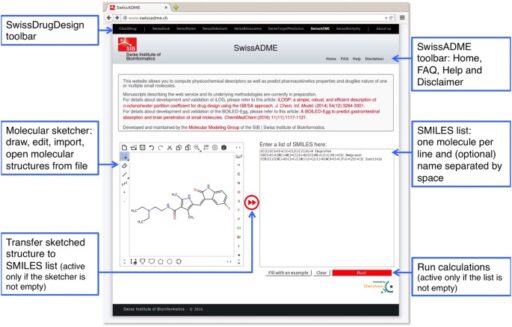
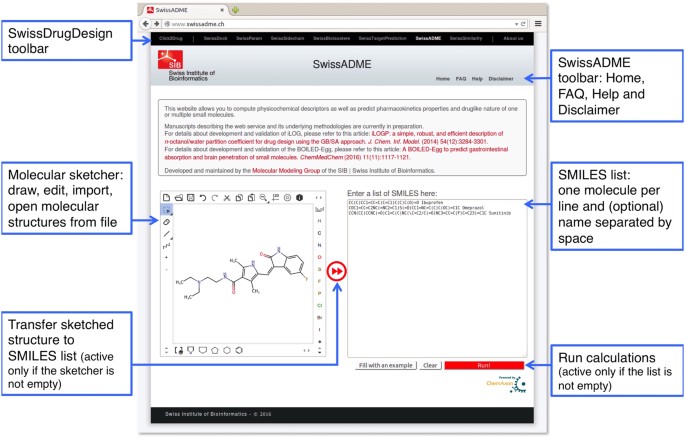
Hay, M., Thomas, D. W., Craighead, J. L., Economides, C. & Rosenthal, J. Clinical development success rates for investigational drugs. Nature Biotechnol. 32, 40–51 (2014).
Dahlin, J. L., Inglese, J. & Walters, M. A. Mitigating risk in academic preclinical drug discovery. Nature Rev. Drug Discov. 14, 279–294 (2015).
Tian, S. et al. The application of in silico drug-likeness predictions in pharmaceutical research. Adv Drug Deliv Rev 86, 2–10 (2015).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 46, 3–26 (2001).
Brenk, R. et al. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 3, 435–444 (2008).
Baell, J. B. & Holloway, G. A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 53, 2719–2740 (2010).
Bruns, R. F. & Watson, I. A. Rules for Identifying Potentially Reactive or Promiscuous Compounds. J. Med. Chem. 55, 9763–9772 (2012).
Irwin, J. J. et al. An Aggregation Advisor for Ligand Discovery. J. Med. Chem. 58, 7076–7087 (2015).
O’Boyle, N. M. et al. OpenBabel: An open chemical toolbox. J. Cheminform. 3, 33 (2011).
Cereto-Massaguà, A. et al. Molecular fingerprint similarity search in virtual screening. Methods 71, 58–63 (2015).
Ertl, P. & Schuffenhauer, A. Estimation of synthetic accessibility score of drug-like molecules based on molecular complexity and fragment contributions. J. Cheminform. 1, 8 (2009).
Clark, A. M. et al. Open Source Bayesian Models. 1. Application to ADME/Tox and Drug Discovery Datasets. J. Chem. Inf. Model. 55, 1231–1245 (2015).
Mitchell, J. B. O. Machine learning methods in chemoinformatics. WIREs Comput. Mol. Sci. 4, 468–481 (2014).
Pires, D. E. V., Blundell, T. L. & Ascher, D. B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 58, 4066–4072 (2015).
Cheng, F. et al. admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 52, 3099–3105 (2012).
Daina, A., Michielin, O. & Zoete, V. iLOGP: A Simple, Robust, and Efficient Description of n-Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 54, 3284–3301 (2014).
Daina, A. & Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 11, 1117–1121 (2016).
Zoete, V., Daina, A., Bovigny, C. & Michielin, O. SwissSimilarity: A Web Tool for Low to Ultra High Throughput Ligand-Based Virtual Screening. J. Chem. Inf. Model. 56, 1399–1404 (2016).
Gfeller, D. et al. SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 42, W32–W38 (2014).
Grosdidier, A., Zoete, V. & Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 39, W270–7 (2011).
Wirth, M., Zoete, V., Michielin, O. & Sauer, W. H. B. SwissBioisostere: a database of molecular replacements for ligand design. Nucleic Acids Res. 41, D1137–43 (2013).
Zoete, V., Cuendet, M. A., Grosdidier, A. & Michielin, O. SwissParam: a fast force field generation tool for small organic molecules. J. Comput. Chem. 32, 2359–2368 (2011).
Ritchie, T. J., Ertl, P. & Lewis, R. The graphical representation of ADME-related molecule properties for medicinal chemists. Drug Discov. Today 16, 65–72 (2011).
Lovering, F., Bikker, J. & Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 52, 6752–6756 (2009).
Ertl, P., Rohde, B. & Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 43, 3714–3717 (2000).
Pliska, V., Testa, B. & van de Waterbeemd, H. In Lipophilicity in Drug Action and Toxicology 1–6 (Wiley-VCH Verlag GmbH, 1996).
Arnott, J. A. & Planey, S. L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 7, 863–875 (2012).
Mannhold, R., Poda, G. I. & Ostermann, C. Calculation of molecular lipophilicity: State‐of‐the‐art and comparison of log P methods on more than 96,000 compounds. J. Pharm. Sci. 98, 861–893 (2009).
Cheng, T. et al. Computation of Octanol−Water Partition Coefficients by Guiding an Additive Model with Knowledge. J Chem Inf Model 47, 2140–2148 (2007).
Wildman, S. A. & Crippen, G. M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Model. 39, 868–873 (1999).
Moriguchi, I., Shuichi, H., Liu, Q., Nakagome, I. & Matsushita, Y. Simple Method of Calculating Octanol/Water Partition Coefficient. Chem. Pharm. Bull. 40, 127–130 (1992).
Moriguchi, I., Shuichi, H., Nakagome, I. & Hirano, H. Comparison of reliability of log P values for Drugs calculated by several methods. Chem. Pharm. Bull. 42, 976–978 (1994).
Ritchie, T. J., Macdonald, S. J. F., Peace, S., Pickett, S. D. & Luscombe, C. N. Increasing small molecule drug developability in sub-optimal chemical space. Med. Chem. Commun. 4, 673 (2013).
Ottaviani, G. et al. What is modulating solubility in simulated intestinal fluids? Eur J Pharm Sci 41, 452–457 (2010).
Savjani, K. T., Gajjar, A. K. & Savjani, J. K. Drug solubility: importance and enhancement techniques. ISRN Pharm 2012, 195727 (2012).
Delaney, J. S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Model. 44, 1000–1005 (2004).
Ali, J., Camilleri, P., Brown, M. B., Hutt, A. J. & Kirton, S. B. Revisiting the general solubility equation: in silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. J. Chem. Inf. Model. 52, 420–428 (2012).
Yalkowsky, S. H. & Valvani, S. C. Solubility and partitioning I: Solubility of nonelectrolytes in water. J Pharm Sci 69, 912–922 (1980).
Potts, R. O. & Guy, R. H. Predicting Skin Permeability. Pharm. Res. 09, 663–669 (1992).
Montanari, F. & Ecker, G. F. Prediction of drug-ABC-transporter interaction–Recent advances and future challenges. Adv. Drug Deliv. Rev. 86, 17–26 (2015).
Szakács, G., Váradi, A., Ozvegy-Laczka, C. & Sarkadi, B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov. Today 13, 379–393 (2008).
Sharom, F. J. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics 9, 105–127 (2008).
Testa, B. & Kraemer, S. D. The Biochemistry of Drug Metabolism – An Introduction – Testa – 2007 – Chemistry & Biodiversity – Wiley Online Library. Chem. Biodivers (2007).
van Waterschoot, R. A. B. & Schinkel, A. H. A critical analysis of the interplay between cytochrome P450 3A and P-glycoprotein: recent insights from knockout and transgenic mice. Pharmacological Reviews 63, 390–410 (2011).
Wolf, C. R., Smith, G. & Smith, R. L. Pharmacogenetics. British Medical Journal (2000).
Di, L. The role of drug metabolizing enzymes in clearance. Expert Opin. Drug Metab. Toxicol. 10, 379–393 (2014).
Hollenberg, P. F. Characteristics and common properties of inhibitors, inducers, and activators of CYP enzymes. Drug Metab. Rev. 34, 17–35 (2002).
Huang, S.-M. et al. New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J. Clin. Pharmacol. 48, 662–670 (2008).
Kirchmair, J. et al. Predicting drug metabolism: experiment and/or computation? Nature Rev. Drug Discov. 14, 387–404 (2015).
Veith, H. et al. Comprehensive characterization of cytochrome P450 isozyme selectivity across chemical libraries. Nature Biotechnol. 27, 1050–1055 (2009).
Cortes, C. & Vapnik, V. Support-vector networks. Mach. Learn. 20, 273–297 (1995).
Mishra, N. K., Agarwal, S. & Raghava, G. P. Prediction of cytochrome P450 isoform responsible for metabolizing a drug molecule. BMC Pharmacol. 10, 8 (2010).
Sedykh, A. et al. Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm. Res. 30, 996–1007 (2015).
Rostkowski, M., Spjuth, O. & Rydberg, P. WhichCyp: prediction of cytochromes P450 inhibition. Bioinformatics 29, 2051–2052 (2013).
Sun, H., Veith, H., Xia, M., Austin, C. P. & Huang, R. Predictive models for cytochrome p450 isozymes based on quantitative high throughput screening data. J. Chem. inf. Model. 51, 2474–2481 (2011).
Carbon-Mangels, M. & Hutter, M. C. Selecting Relevant Descriptors for Classification by Bayesian Estimates: A Comparison with Decision Trees and Support Vector Machines Approaches for Disparate Data Sets. Mol. Inf. 30, 885–895 (2011).
Wang, Z. et al. P-glycoprotein substrate models using support vector machines based on a comprehensive data set. J. Chem. Inf. Model. 51, 1447–1456 (2011).
Ghose, A. K., Viswanadhan, V. N. & Wendoloski, J. J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb. Chem. 1, 55–68 (1999).
Veber, D. F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 (2002).
Egan, W. J., Merz, K. M. & Baldwin, J. J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 43, 3867–3877 (2000).
Muegge, I., Heald, S. L. & Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 44, 1841–1846 (2001).
Martin, Y. C. A Bioavailability Score. J. Med. Chem. 48, 3164–3170 (2005).
Hann, M. M. & Keserű, G. M. Finding the sweet spot: the role of nature and nurture in medicinal chemistry. Nature Rev. Drug Discov. 11, 355–365 (2012).
Teague, S., Davis, A., Leeson, P. & Oprea, T. The Design of Leadlike Combinatorial Libraries. Angew. Chem. Int. Ed. Engl. 38, 3743–3748 (1999).
Fukunishi, Y., Kurosawa, T., Mikami, Y. & Nakamura, H. Prediction of synthetic accessibility based on commercially available compound databases. J Chem Inf Model 54, 3259–3267 (2014).
Zoete, V., Daina, A., Bovigny, C. & Michielin, O. SwissSimilarity. A web tool for low to ultra high-throughput ligand-based virtual screening. J. Chem. Inf. Model. 58, 1399–1404 (2016).
Gfeller, D., Michielin, O. & Zoete, V. SwissSidechain: a molecular and structural database of non-natural sidechains. Nucleic Acids Res. 41, D327–32 (2013).
Lee, M. S., Feig, M., Salsbury, F. R. & Brooks, C. L. New analytic approximation to the standard molecular volume definition and its application to generalized Born calculations. J. Comput. Chem. 24, 1348–1356 (2003).
Brooks, B. R. et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009).
Mak, L. et al. Metrabase: a cheminformatics and bioinformatics database for small molecule transporter data analysis and (Q)SAR modeling. J. Cheminform. 7, 31 (2015).
Poongavanam, V. V., Haider, N. N. & Ecker, G. F. G. Fingerprint-based in silico models for the prediction of P-glycoprotein substrates and inhibitors. Bioorg. Med. Chem. 20, 5388–5395 (2012).
Kim, S. et al. PubChem Substance and Compound databases. Nucleic Acids Res. 44, D1202–13 (2016).
Ward, J. H. Jr. Hierarchical Grouping to Optimize an Objective Function. Journal of the American Statistical Association (1963).
Chang, C.-C. & Lin, C.-J. LIBSVM. ACM Trans. Intell. Syst. Technol. 2, 1–27 (2011).
Egan, W. J. & Lauri, G. Prediction of intestinal permeability. Adv. Drug Deliv. Rev. 54, 273–289 (2002).
Irwin, J. J. & Shoichet, B. K. ZINC – A free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 45, 177–182 (2005).
Cheng, F. et al. Classification of Cytochrome P450 Inhibitors and Noninhibitors Using Combined Classifiers. J. Chem. Inf. Model. 51, 996–1011 (2011).